Phendimetrazine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Bontril |
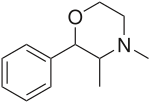
| Other names | Mephenmetrazine; (2S,3S)-3,4-Dimethyl-2-phenylmorpholine |
| AHFS/Drugs.com | Monograph |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Peak plasma levels occur within 1 to 3 hours. Absorption is usually complete by 4 to 6 hours |
| Metabolism | Liver |
| Elimination half-life | 19-24 hours |
| Excretion | Urinary elimination |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.186 |
| Chemical and physical data | |
| Formula | C12H17NO |
| Molar mass | 191.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Image:Phendimetrazine.jpg|thumb|left|Phendimetrazine tablets and capsules
Phendimetrazine (best known by the brand names Bontril, Bontril PDM, Prelu-2 among others, is a central nervous system (CNS) stimulant drug of the morpholine chemical class and effects of a potent appetite suppressant.[2] Indicated as a short-term secondary treatment for exogenous obesity, phendimetrazine was an oral medicine in "pill
in the form of a "pill," either a tablet or capsule, with a common recommendation being for patients to ingest 35mg immediate-release tablet about an hour before meals, up to (but not exceeding) three doses daily.
Brand names
[edit]Phendimetrazine is sold under various brand names, o primarily, but Adipost, Obezine, and Anorex-SR (a particularly (Sustained-Release) formulation comprising a total of 105mg–equivalent to dosing three instant-release tablets over the course of a day, which remains in therapeutic range per the manufacturer's recommendations. The lesser-known and lesser-recognized products were those with generic trade names (e.g. Appecon, Melfiat, Obezine, Phendiet, Plegine, Statobex, TrimTabs), often manufactured by relatively small research laboratories such as Winston Pharmaceuticals, Tutag Pharmaceuticals of New Jersey, Irwin, Neisler & Co., Smith, French & Kline (acquired by GlaxoSmithKline), and Roerig Laboratories (initialy a small-scale manufacturer of primarily nutritional supplements, but acquired and incorporated into Pfizer's marketing division in 1953.
Phendimetrazine was previously manufactured as Anorex-SR, containing in aggregate 105mg of the substance in the dosage form of a Sustained-Release capsule for once daily dosing, typically consumed 30 to 60 minutes before a morning meal. Whereas the immediate-release formulation has a maximum daily dosage of 210mg (6 tablets), the extended-release capsules have a maximum daily dosage of 105mg (one capsule).
Mechanism of Action
[edit]Phendimetrazine is a prodrug of phenmetrazine, which is an amphetamine derivative and at the time was a commonly-prescribed weight loss medication known by its brand name Preludin, which was first released in the 1950s, but was discontinued in 1982 due to widespread misuse and increased scrutiny and enforcement of violations involving controlled substances. The launch of Bontril and Bontril PDM in 1972; approximately 30 percent of an oral dose phendimetrazine is converted into phenmetrazine. As with any prodrug, the conversion process is bound by the timeframe of the process of drug metabolism, hence it is effectively an extended-release formulation of a relatively low dose of phenmetrazine; consequently having a lower abuse potential. Phendimetrazine is an anorectic drug which acts as a norepinephrine-dopamine releasing agent (NDRA).[3]
Pharmacology
[edit]As an amphetamine congener, its structure incorporates the backbone of methamphetamine, a potent central nervous system stimulant. While the addition of an N-methyl group to amphetamine significantly increases its potency and bioavailability, methylation of phenmetrazine renders the compound virtually inactive. However, phendimetrazine is a prodrug for phenmetrazine which acts as the active metabolite. Phendimetrazine possesses preferable pharmacokinetics over phenmetrazine as a therapeutic agent because its metabolization by demethylases produces a more steady and prolonged exposure of active drug within the body. This decreases abuse potential as the peak blood-concentration of active phenmetrazine that's produced from a single dose of phendimetrazine is lower than a single therapeutically equivalent dose of phenmetrazine.
Chemistry and Synthesis
[edit]The reaction between N-methylethanolamine and 2-bromopropiophenone gives compound (3), which is reductively cyclized using formic acid to synthesize phendimetrazine.[4][5]
Legality, Regulation, and Classification as a Controlled Substance
[edit]According to the List of Psychotropic Substances under International Control published by the International Narcotics Control Board, phendimetrazine is a Schedule III controlled substance under the Convention on Psychotropic Substances.[6] At the national level, U.S. correlates to the convention's ruling. The Controlled Substances Act of 1970 classifies the substance as Schedule III, alongside many anabolic steroids.
References
[edit]- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ Landau D, Jackson J, Gonzalez G (2008). "A case of demand ischemia from phendimetrazine". Cases J. 1 (1): 105. doi:10.1186/1757-1626-1-105. PMC 2531092. PMID 18710555.
- ^ Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–59. doi:10.2174/156802606778249766. PMID 17017961. Retrieved 5 May 2020.
- ^ "Phendimetrazine". Thieme. Retrieved 30 June 2024.
- ^ Werner Heel and Karl Zeile, U.S. patent 2,997,469 (1961 to Ingelheim, Germany, assignors to C. H. Boehringer Sohn, Ingelheim, Germany, a partnership).
- ^ "List of psychotropic substances under international control" (PDF). Archived from the original (PDF) on 31 August 2012. Retrieved 15 June 2005.

